MAKE PROTECTION
YOUR SUPERPOWER

See how SYNJARDY® driven by Empagliflozin offers multiple metabolic benefits.*1-3
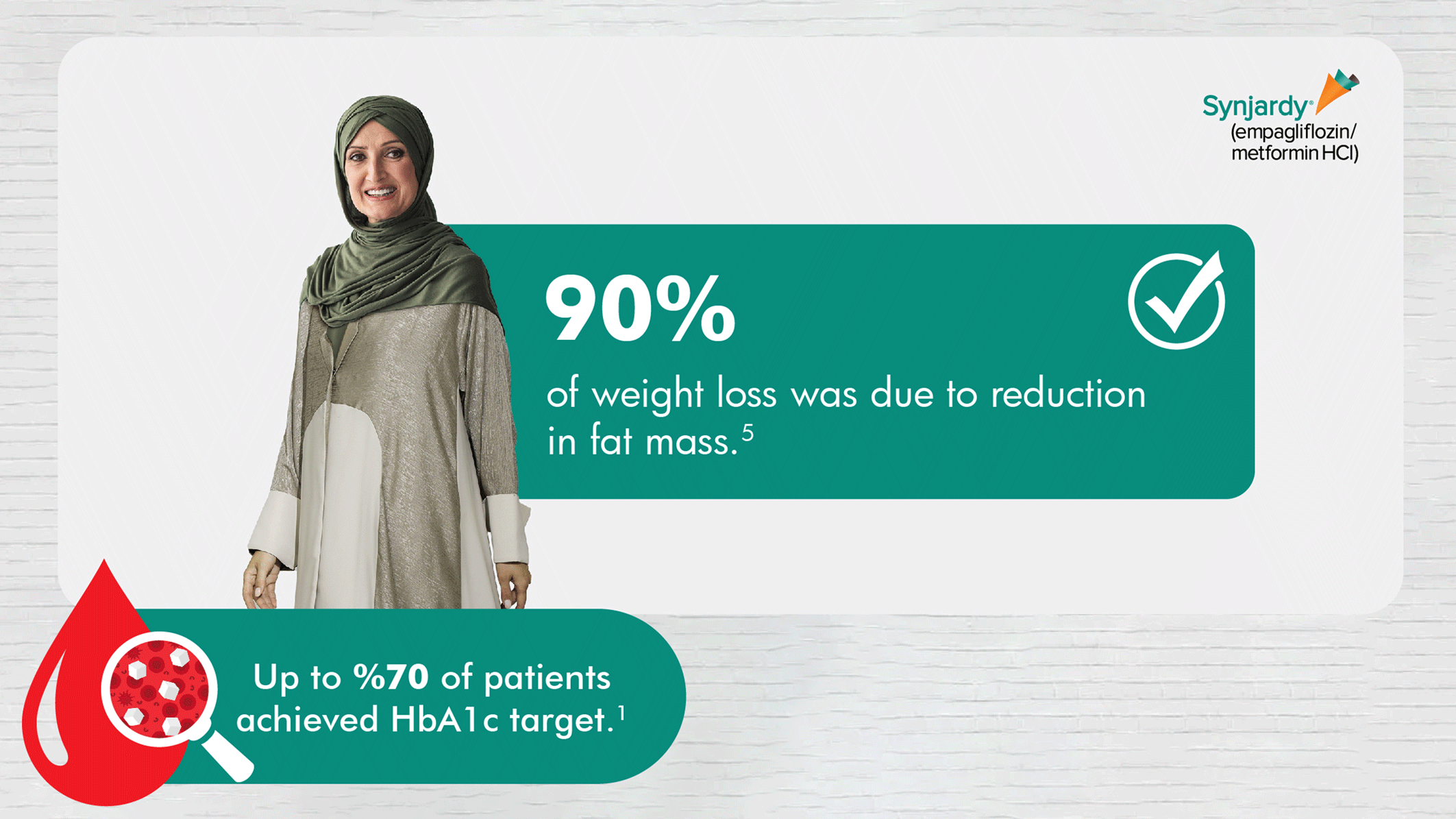

SYNJARDY® isn't indicated for weight loss
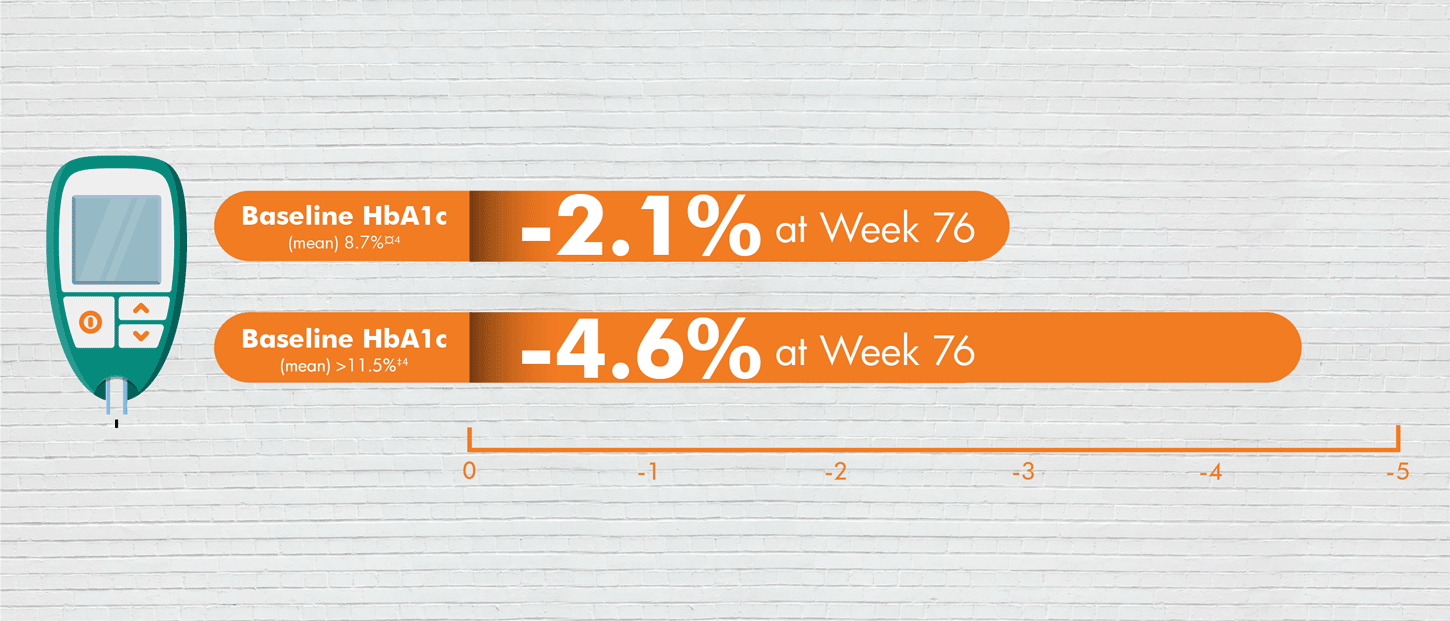
*In a 24-week, double-blind, placebo-controlled study of 637 patients with type 2 diabetes, the efficacy and safety of empagliflozin 10 mg (n=217) and empagliflozin 25 mg (n=213) as add-on therapy to metformin ≥1500 mg were evaluated versus placebo added metformin (n=207). The primary endpoint was adjusted mean change (SE) from baseline in HbA1c (%); weight loss and blood pressure reduction were key secondary and exploratory endpoints, respectively. ¤Adjusted mean changes of -0.1% from baseline 7.9% for placebo (n=07), -0.7% from baseline 7.9% for Empagliflozin 10 mg (n=217), and -0.8% for Empagliflozin 25 mg (n=213). Difference from placebo (adjusted mean) was -0.6% for both Empagliflozin 10 and 25 mg; P<0.0001 versus placebo for both doses. ‡In a subgroup analysis at 24 weeks, of patients with baseline ≥8.5%, adjusted mean changes in HbA1c were -0.5% for placebo (n=50), -1.2% for Empagliflozin 10 mg (n=57), and -1.5% for Empagliflozin 25 mg (n=48). Difference from placebo (adjusted mean) was -0.73% for Empagliflozin 10 mg and -1.0% for Empagliflozin 25 mg. §Adjusted mean changes of -0.45 kg reduction in body weight from baseline 79.7 kg for placebo (n=207), -2.08 kg from baseline 81.6 kg for Empagliflozin 10 mg (n=217), and -2.46 kg from baseline 82.2 kg for Empagliflozin 25 mg; P<0.001 versus placebo for both doses. ‖In a subgroup analysis at 24 weeks, of patients with baseline BMI ≥35 adjusted mean changes in weight were -0.34 kg for placebo (n=29), -2.63 kg for Empagliflozin 10 mg (n=33), and -3.35 kg for Empagliflozin 25 mg (n=41). Difference from placebo (adjusted mean) was -2.28 for Empagliflozin 10 mg and -3.01 for Empagliflozin 25 mg. In a double-blind extension trial, adjusted mean changes in weight for patients with baseline BMI ≥35 at Week 76 were 0.23 kg for placebo (n=29), -3.74 kg for Empagliflozin 10 mg (n=33), and -4.77 kg for Empagliflozin 25 mg (n=41). Difference from placebo (adjusted mean) was -3.96 kg for Empagliflozin 10 mg and -4.99 for Empagliflozin 25 mg.
References:
-
1.
Hadjadj, S., Rosenstock, J., Meinicke, T., Woerle, H. J., & Broedl, U. C. (2016). Initial Combination of Empagliflozin and Metformin in Patients With Type 2 Diabetes. Diabetes care, 39(10), 1718-1728. https://doi.org/10.2337/dc16-0522
-
2.
Local SMPC Empagliflozin (Summary of Product Characteristics). July 2023.
-
3.
Data on file. Boehringer Ingelheim Pharmaceuticals, Inc.
-
4.
Häring HU, Merker L, Seewaldt-Becker E, et al; EMPA-REG MET Trial Investigators. as add-on to metformin in type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2014;37(6): 1650-1659.
-
5.
Ridderstrale M, Andersen KR, Zeller C, Kim G, Woerle H, Broedl U; EMPA-REG H2H-SU Trial Investigators. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2(9):691-700.
PC-AE-102267 | Expiry Date: 10/08/2026
