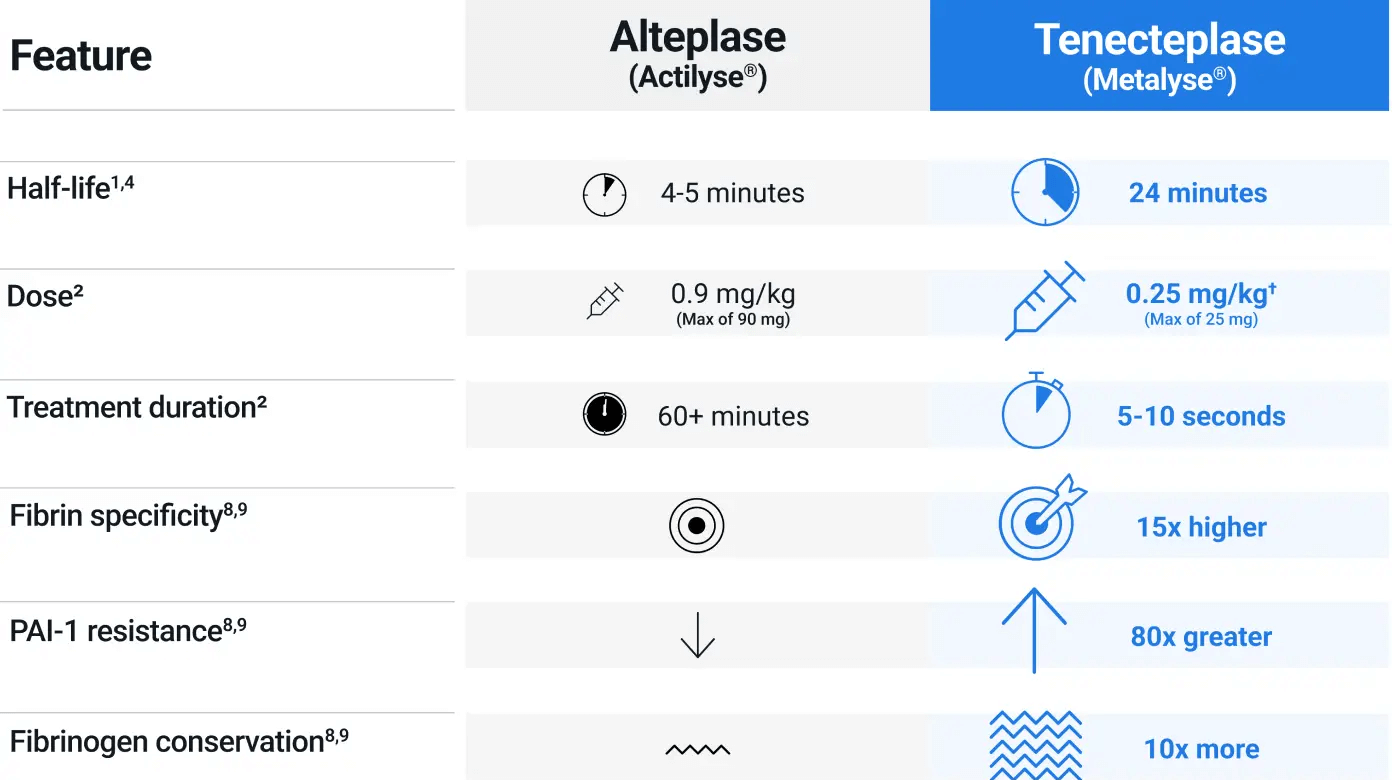
Single IV bolus administration
Metalyse® 25 mg is administered by a single IV bolus over 5 to 10 seconds, eliminating the need for a one-hour infusion, as required for Actilyse®1-3
- Metalyse® 25
Metalyse® 25 mg comes as one single vial of powder for reconstitution, regardless of the patient
The dosing for Metalyse® 25 mg is simple with only 5 weight-based tiers:
| Weight (kg) | Volume (ml) | Tenecteplase (U) | Tenecteplase (mg) |
|---|---|---|---|
| < 60 | 3 | 3000 | 15 |
| ≥ 60 to < 70 | 3.5 | 3500 | 17.5 |
| ≥ 70 to < 80 | 4 | 4000 | 20 |
| ≥ 80 to <90 | 4.5 | 4500 | 22.5 |
| ≥ 90 | 5 | 5000 | 25 |

PDF Document for Preparation and Administration

Video for Preparation and Administration
Footnotes
-
IV: intravenous; PAI-1: plasminogen activator inhibitor-1
-
*
One of the common contributing factors that led to dosing errors associated with alteplase administration was the infusion pump being programmed incorrectly. Additionally, a single IV bolus administration avoids the interruption of treatment, further reducing dosing errors.
-
†
Tenecteplase was administrated within 4.5 hours after onset of stroke symptoms, as a weight-tiered bolus dose, based on 0.25 mg/kg by 10 kg steps, for the maximum weight at each tier: < 60 kg, 15 mg tenecteplase; ≥ 60 to < 70 kg, 17.5 mg; ≥ 70 to < 80 kg, 20 mg; ≥ 80 to < 90 kg, 22.5 mg; and ≥ 90 kg, 25 mg.
References
-
1.
Metalyse® Summary of Product Characteristics, January 2024.
-
2.
Menon BK, et al. Lancet 2022;400:161-169.
-
3.
Bivard A, et al. Lancet Neurol. 2022;21:520-27.
-
4.
Actilyse® European Summary of Product Characteristics.
-
5.
Dancsecs K. A. et al. Am J Emerg Med. 2021;47:90-94.
-
6.
Miller, SE and Warach, SJ. Neurotherapeutics 2023;20:664–678.
-
7.
Zhu A, et al. Res Pract Thromb Haemost. 2022;6:e12795.
-
8.
Huang X, et al. Stroke. 2015;46(12):3543–6.
-
9.
Tanswell P, et al. Clin Pharmacokinet. 2002;41(15):1229–45.
PC-AE-102393 | Expiry Date: 02/18/2029